
There’s a unity to life. Sometimes it’s plain to see, but very often it lurks underneath a distraction of differences. And a new study shows that there’s even a hidden unity between our slipped disks and the muscles in a squirming worm.
Scientists call this unity “homology.” The British anatomist Richard Owen coined the term in 1843, sixteen years before Charles Darwin published The Origin of Species. Owen defined homology as “the same organ in different animals under every variety of form and function.” For example, a human arm, a seal flipper, and a bat wing all have the same basic skeletal layout. They consist of a single long bone, a bending joint, two more long bones, a cluster of small bones, and a set of five digits. The size and shape of each bone may differ, but the pattern is the same regardless of how mammals use their limbs–to swim, to fly, or to wield a hammer.
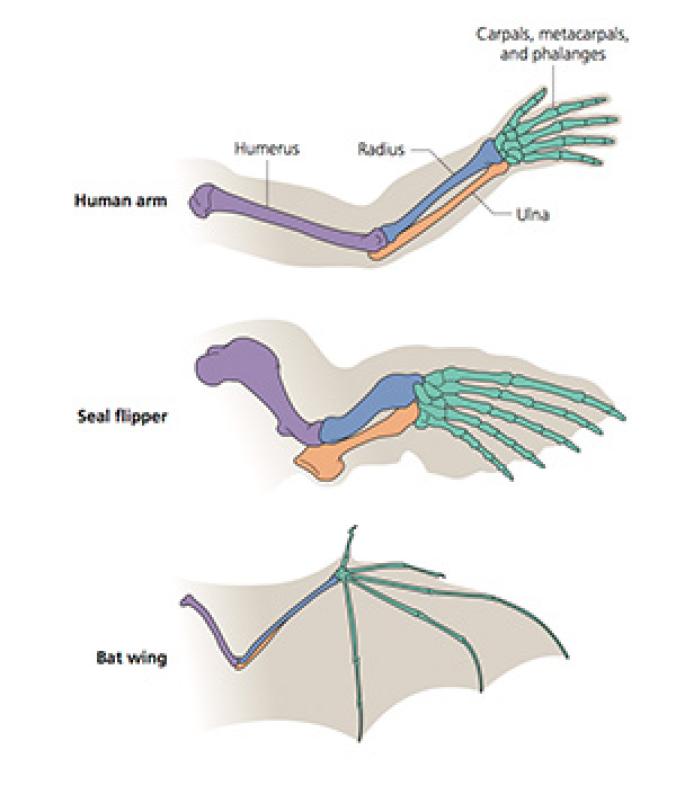
Darwin argued that homology was the result of evolution. The common ancestor of humans, seals, bats, and other mammals had a limb which became stretched and squashed in various contortions. And over the past 150 years, paleontologists have found a wealth of fossils that help document how the tiny paws of Mesozoic mammals diversified into the many forms found in mammals today.
But Darwin wasn’t just out to explain the evolution of mammals. He saw a kinship across the entire living world. And that’s where things got complicated. Anatomists in Darwin’s day could find no clear counterparts to many of the traits in our own bodies in distantly related animals.
It turns out the homology is there, but you just need the right eyeglasses to see it.
Recently, Detlev Arendt, a biologist at the European Molecular Biology Laboratory, and his colleagues investigated the evolution of an important but overlooked feature in our bodies, known as the notochord. It’s a stiff rod of cartilage that develops in human embryos, running down their back. Later, as the spine develops, the notochord transforms into the disks that cushion the vertebrae (and sometimes slip later in life, causing much grief).
Other mammals also develop a notochord as embryos. And so do birds, reptiles, amphibians, and fish. Even our closest invertebrate relatives, such as lancelets, have notochords. All animals with a notochord belong to the same group, known as the chordates.
Unlike most vertebrates, lancelets keep their notochord into adulthood, using it to stiffen their bodies when they swim. Early chordate fossils also have a lancelet-like anatomy. So it’s likely that 550 million years ago, the notochord evolved in chordates first, and then the skeleton evolved later. In fish, the spine took over the body-stiffening job, but the notochord still had other work left to do. In the vertebrate embryo, the notochord releases chemical signals that tell the surrounding cells whether they should become nerves, blood vessels, or other tissues.
Arendt and his colleagues wondered how the notochord first evolved. Squid don’t have a notochord. Neither do clams, or cockroaches, or tarantulas. The notochord, in other words, seems to be unique to chordates. So where did it come from? Did it emerge right at the dawn of chordates, or did it have deeper origins?
The scientists decided to tackle these questions by looking at the genes in notochord cells. In a developing vertebrate embryo, notochord cells switch on a unique combination of genes. The scientists wondered if the genetic signature of a notochord cell might be lurking in animals that have no notochord.
They started their search in ragworms, ocean-dwelling relatives of the more familiar earthworms. Worms (or to be more precise, annelids) are an ancient lineage that split off from our own ancestors long before the notochord evolved. There’s nothing in their squishy bodies that you would mistake for a notochord.
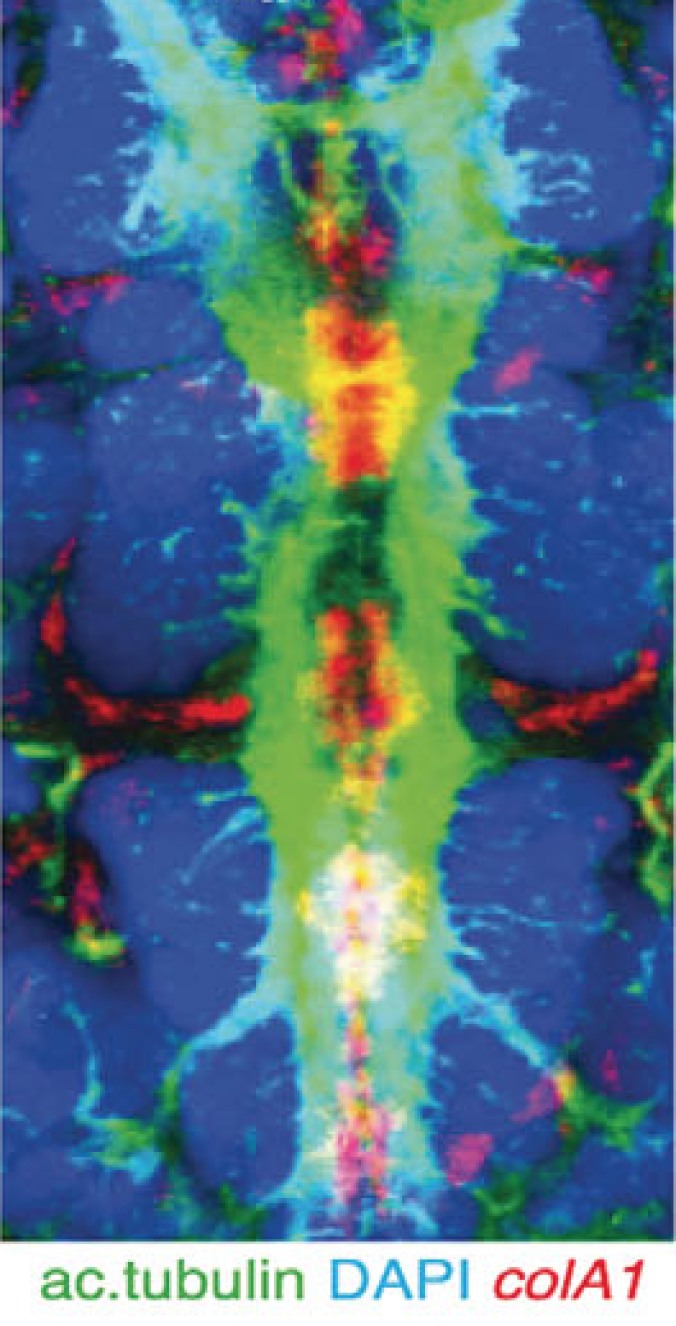
Arendt and his colleagues added chemicals to ragworm larvae to make cells glow if they were using notochord genes. The larvae lit up like Christmas trees.
The cells using notochord genes formed a strip running from the head to the tail of the ragworm larvae–in much the same arrangement as our own notochord. When the rag worm larvae matured, the scientists found, the strip developed into a cord of muscle.
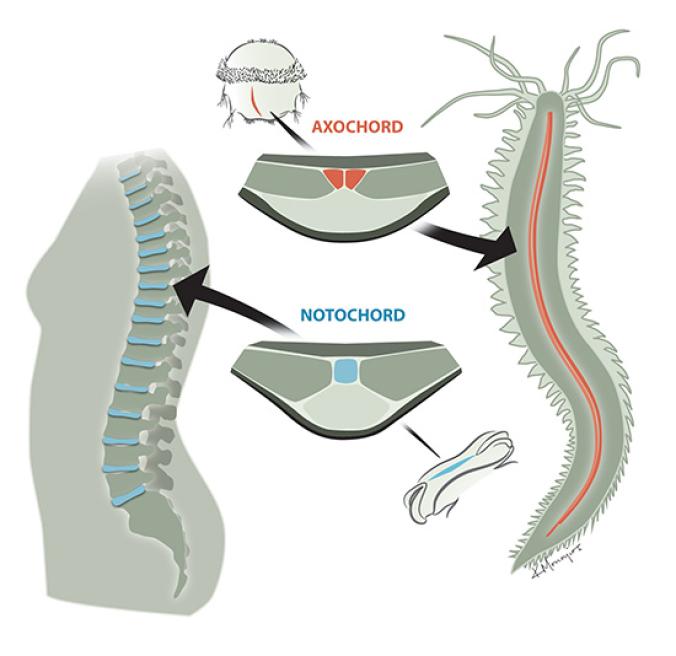
The worms need this cord–which the scientists dubbed the axochord–to move around. When the scientists destroyed the axochord with a laser, the ragworms could no longer swim. And once Arendt and his colleagues discovered the axochord in ragworms, they looked for it in other animals that lack a notochord. They found signs of axochords in a number of other invertebrates.
This study reveals the homology of notochords and axochords, but it also does something more. It helps us go back in time. Ragworms and humans share a common ancestor, along with other animals that have brains, heads, tails, and distinct left and right sides. Collectively all these animals are known as bilaterians. The emergence of bilaterians some 700 million years ago was a tremendous evolutionary event, giving rise to a huge diversity of animal forms and even changing the chemistry of the oceans and atmosphere.
Evolutionary biologists would love to know what the first bilaterians looked like, and to understand how they gave rise to such different animals today. One of the biggest surprises in the history of biology has been the discovery that bilaterians share a deep homology in the genes that build their bodies. Fly eyes and human eyes may look different, for example, but the same network of genes helps build both kinds. Or take the front and back of our bodies. In us (and other chordates), the main nerve cord runs down the back and our digestive tract runs down the front. In a fly or in many other bilaterians, the arrangement is vice-versa. But all bilaterians use the same genes to tell the two sides apart.
These discoveries let scientists develop hypotheses about what the first bilaterians looked like. They may have already had a head, tail, brain, and eye-like senses for example. And the new study hints that they may have had a precursor of our notochord. Our own cartilage notochord turns out to be a peculiar variation on the bilaterian body plan. Other bilaterians have an axochord–basically, a notochord made of muscle. So it’s possible that 700 million years ago, our first bilaterian ancestors had an axochord made of muscle, which they might have used to swim. The descendants of these first bilaterians diverged into many body forms. In a few lineages, Arendt and his colleagues argue, the axochord was transformed into structures that look dramatically different today. We belong to one of those lineages.
The new study suggests that the signals that our notochord cells received changed slightly, switching from muscle to cartilage. This is actually easier than it may sound, since embryonic stem cells are incredibly versatile. In fact, we can fall victim today to such anatomical mix-ups. Last year, for example, I wrote a story for The Atlantica story for The Atlantic about a rare disease called FOP that switches muscles to cartilage, which then turns to bone. It takes a single mutation to produce this disease.
Only 1 in 2 million people get FOP. Slipped disks are a far more common disorder. As many as a third of people may end up with a disk bulging out of place. That’s the kind of risk you run when your ancestors’ notochord evolves into spine cushions, and then, much later, your ancestors start walking around upright. If you ever find yourself laid up in bed because your notochord fails you, try distracting yourself by reflecting on its long evolutionary history reaching back over half a billion years, and the unity it shares with worms wriggling through the sea.
(Here’s a video showing the axochord in 3-D)
(Update: changed title to better reflect story)